In Chemistry, every matter, whether it’s in solid, liquid or gaseous state is affected by a certain law. There are various laws concerning gravity, motion, etc. And those laws are mostly being credited to the one who had made some research, therefore coining the term ‘discovery’. And Avogadro’s Law is no exemption.
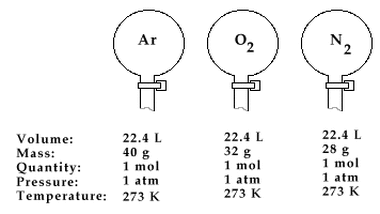
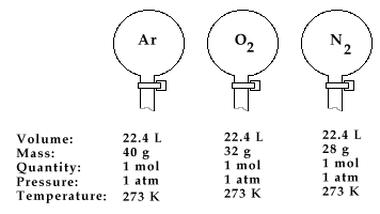
This law, also known as Avogadro’s Theory or Hypothesis (meaning an educated guess), was described by an Italian Physicist Amedeo Avogadro in 1811. This law, according to him, is the relation which states that at the same pressure and temperature, equal volumes of gases contain the same number of molecules. In order to make things clear, even though gas molecules are known to be in a dispersed state, when put in a container and has undergone a standard temperature and pressure, their number can be somehow calculated.
In terms of mathematical equation, this law is expressed as k=V/n, where k is a proportionality constant, V is the gas volume itself while the n is the number of moles (or mol) of that gas. Moles, is the standard measure when it comes to measuring great quantities of very small particles such as atoms and molecules.
According to Avogadro, the ideal gas constant’s value is always the same for all ideal gases so it can also be expressed like this:
Constant = P1V1/T1n1 = P2V2/T2n2, V1/n1 = V2/n2 or V1n2 = V2n1, whereas in this equation, P stands for gas pressure and T stands for temperature.
Therefore, if we’re going to apply this law, it will suggest that the molar volume of all ideal gases at 0°C is 22.4 liters. Also take note that when the amount of gas increases, the volume also increases since they are both proportionality constant.