You might find yourself in a Chemistry assignment with your teacher requiring you to compute or get to know the number of electrons, protons and neutrons of an atom from the different kinds of elements. In order to do this, you must first acquire a Periodic Table or the Table of Elements. Fortunately, this one-page table is available just over the internet. You can print it out or simply buy it from any school supply center. If you already have, take these following steps as your guide for computation:
- For the basics, remember the following equations by memorizing them:
- of Protons = Atomic No. of Element
- of Electrons = No. of Protons
- of Neutrons = Mass No. – Atomic No.
- Finding the Number of Protons
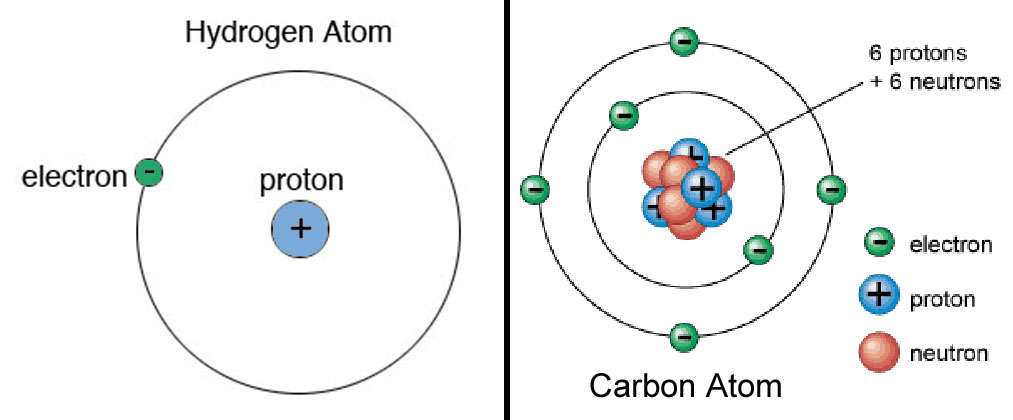
- An element is always defined by the total no. of protons within its atom. Hence, the proton dictates the atomic number of an element. If you can notice, the elements within the Periodic Table are arrayed based on the increasing scale of atomic number. Remember the first equation mentioned in Step 1: No. of Protons = Atomic No. of Element.
- Example: Hydrogen (H) proton number is 1; hence the atomic number of Hydrogen is also 1.
Zinc proton number is 30, so the atomic number of this element is also 30.
- Finding the Number of Electrons
- Remember: Neutral atoms reflect that both electrons and protons bear the same number. However, as most atoms have positive or negative charge, you are left to do some calculations.
- You can know the number of electrons in an ion after knowing its charge.
- Cation- bears more protons than electrons, so it has positive charge.
- Anion- bears more electrons than protons, so it has negative charge.
- Neutron- does not bear a net of electric charge.
- The number of protons in an atom is not changeable so you can add or subtract electrons to get the charge.
- Example: Zn2+ (meaning the ion has exceeded 2 protons over the number of electrons.)
30 (Atomic no. of Zinc) — 2 = 28 electrons.
- Finding the number of Neutrons
- Find first the mass number of each element. Mass number differs from the atomic weight indicated in the periodic table. However, you can use the atomic weight of the element to find its mass number. The atomic weight of an element is expressed into decimal number. Round it off to the nearest whole number.
- Example: Hydrogen has an atomic weight of 1.008. It’s nearest to the whole number 1 than 2, so use 1 instead of 2.
- Using the formula: No. of Neutrons = Mass Number – Number of Protons, that is 1—1 = 0