When you’re studying Chemistry, these are the laws that you have to take note.
- Avogadro’s Law – Ideal gases under equal temperature and pressure conditions will also contain equal number of particles, whether it is molecules, atoms, electrons, etc.
- Boyle’s Law – The confined gases’ volume is inversely proportional to the pressure at constant temperature.
- Charles’ Law – The confined gas volume is directly proportional to the absolute temperature at constant pressure.
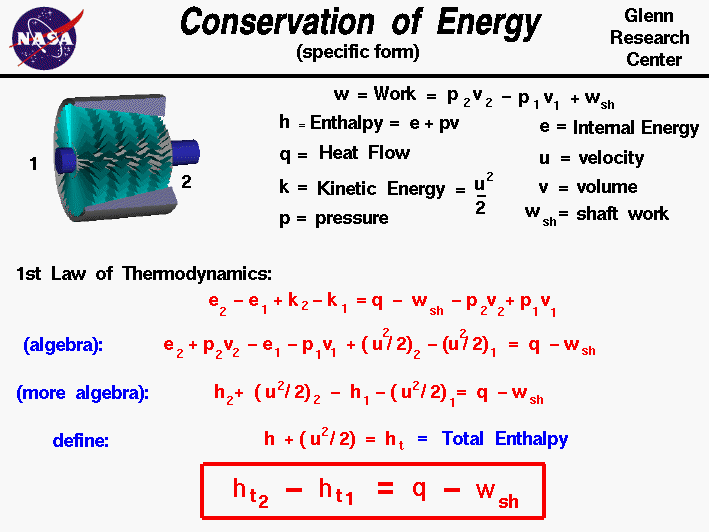
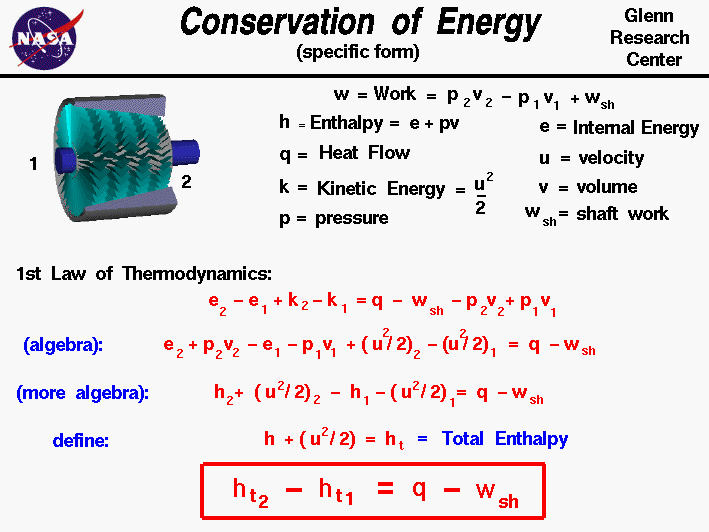
- Conservation of Energy – Being the First Law of Thermodynamics, it states that energy can be neither created nor destroyed, therefore making the universal energy constant.
- Conservation of Mass – Matter can be neither created nor destroyed, though it can undergo chemical changes while the mass, however, remains the same.
- Dalton’s Law – The gases mixture’s pressure is equal to the sum of component gases’ partial pressures.
- Definite Composition – Two or more elements of a definite weight chemically combined are needed to form a compound.
- Dulong and Petit’s Law – In most metals, 6.2 calories are needed to raise the temperature of 1g by 1 degree centigrade.
- Faraday’s Law – The weight of any element during electrolysis is proportional to the electricity’s quantity and to the element’s weight.
- Gay-Lussac’s Law – The ratio between the gas volumes and product combined can be expressed in whole numbers.
- Graham’s Law – The diffusion or effusion rate of a gas is inversely proportional to its molecular mass’ square root.
- Henry’s Law – The gas’ solubility is directly proportional to the applied pressure, unless it’s highly soluble.
- Ideal Gas Law – The ideal gas’ state is determined by volume, pressure and temperature.
- Multiple Proportions – The mass of one element combined with a fixed mass of another, is done in a small whole number-ratio system.
- Periodic Law – Every element’s chemical property varies according to their atomic number.
- Second Law of Thermodynamics – Heat cannot flow on its own from cold to a hot area.